Atoms, Molecules, & Equations
Use the links to atoms and the periodic table below to help you with the handout.

Brainpop: Atoms
Atoms are organized in a chart (the periodic table) based upon the number of tiny parts each one has. Tim and Moby magically go inside atoms to show you the relationship of the tiny moving parts. And get hit by them.
When you are learning how to cook something new, it helps to look at a recipe. Here you can see that the person mixing cookie dough has some of the ingredients listed in the recipe next to the mixing bowl.
Parts of the recipe will use code. For example, teaspoon = t , tablespoon = T, and cups = C. A similar code exists for elements. Each element can be represented by just one or two letters. Numbers are then used to indicate how many of each atom is being used.
| |
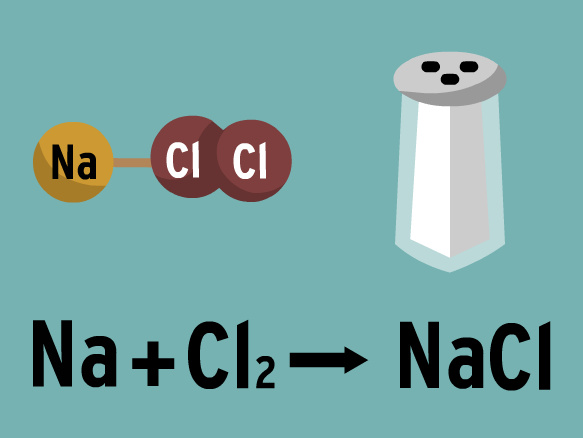
Brainpop: Chemical Equations
Tim and Moby help to explain the different chemical symbol codes. Moby is naughty and drinks strange things.
Think about how a see-saw works. Unless there is the same amount of weight on each end, it will not balance perfectly. The same thing applies to chemical equations. If there are not the same number of atoms on each side, the equation is not balanced. This is because atoms cannot be made to magically appear or disappear (Conservation of Mass). However, they can be arranged in different combinations to make different molecules.
| |
Brainpop: Conservation of Mass
Tim and Moby learn how "No" applies to things in chemistry. The difference between mass and weight is explained. And, after all the explaining, they fail to turn lead into gold, much to Moby's disappointment.


