States of Matter

Everything living needs water to survive. If water is not immediately available, organisms figure out ways to get it. This could include collecting dew during the night, like some desert plants, or eating animals and absorbing the water from their blood.
| |
Brainpop: Water
Tim talks about how much he likes water. Moby helps him to like it a little less.
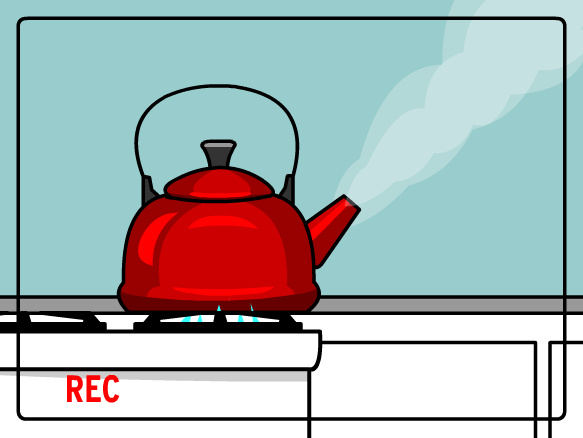
You can see that the kettle is on a stove, and the burner is turned on under the kettle. Blue flame shows that energy (heat) is going from the stove to the kettle. The energy passes through the metal of the kettle to the water inside. After a bit of time, the water begins to boil, and you hear a whistle as steam escapes out a hole in the cover over the spout.
You will use this image and the ones below to analyze what happens when you add energy to water, or take energy away from it. Handout below.
| |
| |
| |
Another example of energy being added to water is here in Yellowstone Park. Volcanic activity (lots of magma underground, fairly close to the surface) heats up groundwater to the point of actually boiling. The bubbles and steam you see here are just as dangerous as those made by the pot of boiling water on your stove.
| |
| |
| |
| |

How solids, liquids, and gases behave all comes down to how closely attracted the particles in the material are to each other. In a solid, each particle is touching other particles, so it is able to keep its shape until something comes along (like a jackhammer) that is able to force the particles apart.
Think of the particles in a solid to be acting like students in a classroom. Students are not perfectly still, but wiggling around in their assigned seats.
| |
A person competing in the belly-flop contest is very thankful that the particles in a liquid are not locked together like those in a solid. Otherwise, instead of making a splash, they would make a squish. They would only compete ... once. Because the particles of a liquid can move around, the body of the person hitting the water is able to move the particles of liquid aside, making space for the body in the pool.
Think of the particles in a liquid to be acting like students in the hallways between classes. They are moving in a certain direction, but slowly, and bumping into each other a lot.
| |
The space between particles in a gas can vary, depending upon how hot the gas is. The more heat that is added, the farther apart the particles get. Add heat to the gas inside a balloon, the particles spread farther apart, and the balloon goes up. The opposite is true when the gas cools down - the balloon begins to sink. So... gas inside a balloon (the fabric is solid) moves the balloon up and down in a gas (the air).
Particles in a gas move like students at a free play day at P.E. Quickly, all over the place, and in no particular direction.
| |
Brainpop: States of Matter
Adding energy, removing energy, and the zoom function of a camera gets out of control.
| |
| |
| |
Nope, this is not a doctored photo. These Japanese monkeys have actually figured out that sitting in hot springs while it is cold outside is a great way to stay warm. So many different states of matter in the picture, too! Steam, air, water, rocks....
You'll be exploring the different properties of states of matter in the lab below. Including trying to make a zombie ooze through your table.
| |

Candy corn. You either love it or hate it. In this instance, we'll ignore your personal feelings about it and instead use it to introduce a way of thinking about particles in a state of matter. Oddly enough, this is done by thinking about how the particles of a substance would behave if they were trapped in a jar.
Notice how the candy corn is keeping its shape and not moving around. There are also small spaces between the candy corn particles. The behavior and spacing of these particles in the jar is representative of particles in a solid.
| |
Moving on to fish in a jar. As the fish need water to survive, the jar is filled with water. As the fish swim around, the particles of the water are pushed out of the way because they are not as tightly connected as particles of a solid. The fish counts as a solid, because it keeps its fishy shape the whole time it is alive. Note that the water also fills the jar out to the sides of the container. However, the water may not fill the jar all the way to the top. Turn the jar over, and whatever air was between the water surface and the lid of the jar will move around the jar.
| |
Just as the fish need an oxygen source (water), the insects in this jar need an oxygen source as well (gas). Just like contents of the jar filled with fish and water, the insects here are able to move around in the jar, their solid bodies pushing the particles of air around. However, unlike the jar with water, that is only filled up to a certain point, the jar of air remains completely full of the gas. If you turn it upside down, the insects will get irritated, but there won't be a small bubble of air moving around, like it could in the water jar.
| |
And of course, every good scientist needs a brain in a jar to study. As anatomists started studying the human body, poking around to see what was inside, they figured out ways for organs to be preserved. Once preserved, they could be compared with brains from other people or other animals.
Some people believed very strongly at the time (and some still do) that the human body should not be opened up. This belief, combined with scientists exploring what it meant to be alive, gave rise to the classic book "Frankenstein", by Mary Shelly. In the story, Dr. Frankenstein uses the brain from another person to put inside his patchwork monster, then brings it to life with electricity.
| |

This still is from the movie Young Frankenstein (1974). This movie takes a more humorous look at the Frankenstein story (even using the laboratory props from the original movie!). In this particular scene, Dr. Frankenstein's assistant Igor has already dropped the brain he was sent to get. He is now considering a substitute. What could possibly go wrong if they used an abnormal brain for their experiment?
| |
Click on the ACS logo below to access pages that will help you with an assignment that will help you learn how particles in different states of matter look and interact.

| |

| |
| |
| |
This assignment will describe the characteristics of different states of matter, and ask you to identify them as describing a solid, a liquid, or a gas. You will find it helpful to use the following links:
You may have experienced a different kind of change in your body when the weather suddenly gets colder than you dressed for. As the warmth (energy) goes away, you find yourself covered in goose bumps. Your body is trying to get you warmer by fluffing your fur up for insulation. Except you don't have long thick fur, except perhaps on your head.
Birds also have a way to try and get warmer. They fluff up their feathers, trapping a layer of air which acts as insulation.
All of this is to make you think about physical changes that happen when heat is added to or removed from an animal's body. This change in heat (energy) prompts a visible response by your body, and you then look wetter or bumpier than you did before. When energy is added or removed from a substance, it can look different as well. Think of adding energy to ice, and it melts. Or, removing energy from water, and it hardens into ice.
| |
Brainpop: Matter Changing States
Tim has to really keep on his toes, as Moby is determined to get him with either a squirt gun or ice cubes down the pants. All in the name of science, right?
When working through the handout on this Brainpop, you may also find it helpful to review the matter overview from chem4kids linked below.
| |

Brainpop: Water Cycle
Tim explains things while sitting in a bathtub. Moby gets jealous of the water cycle.
| |
The text link below will take you to an interactive diagram about the water cycle. The handout that goes with it has been designed to work best with the "intermediate" diagram. You can certainly explore other versions of it, but "intermediate" will work best for the assignment.
Before freaking out because a diagram of different states of matter can look complicated, think about a rock climber for a minute. The higher the climber gets on the rock face, the more energy they have used, right?
The same thing applies to states of matter diagrams. The higher you get on the diagram, the more energy has been used to change from one state to another.
The rock climber analogy works for moving from the left to the right on a states of matter diagram as well. You can easily understand that to climb high takes a long time, as well as a lot of energy. So, as more time passes (going left to right on the diagram), more energy is needed (going up on the diagram).
| |
This exercise will help you to think logically about how particles move in different states of matter. You'll also get some tips on how to show different amounts of movement in your drawings of particles.
There are lots of different ways that graphs can be used to show information about states of matter. You can examine temperature, movement, energy, drawings, time, etc. So many things! You'll learn how to slow down and not panic, instead looking carefully at graph labels and, once again, thinking logically.


























